Summary
Background
Global dietary patterns are increasingly dominated by relatively cheap, highly palatable, and ready-to-eat ultra-processed foods (UPFs). However, prospective evidence is limited on cancer development and mortality in relation to UPF consumption. This study examines associations between UPF consumption and risk of cancer and associated mortality for 34 site-specific cancers in a large cohort of British adults.
Methods
This study included a prospective cohort of UK Biobank participants (aged 40–69 years) who completed 24-h dietary recalls between 2009 and 2012 (N = 197426, 54.6% women) and were followed up until Jan 31, 2021. Food items consumed were categorised according to their degree of food processing using the NOVA food classification system. Individuals’ UPF consumption was expressed as a percentage of total food intake (g/day). Prospective associations were assessed using multivariable Cox proportional hazards models adjusted for baseline socio-demographic characteristics, smoking status, physical activity, body mass index, alcohol and total energy intake.
Findings
The mean UPF consumption was 22.9% (SD 13.3%) in the total diet. During a median follow-up time of 9.8 years, 15,921 individuals developed cancer and 4009 cancer-related deaths occurred. Every 10 percentage points increment in UPF consumption was associated with an increased incidence of overall (hazard ratio, 1.02; 95% CI, 1.01–1.04) and specifically ovarian (1.19; 1.08–1.30) cancer. Furthermore, every 10 percentage points increment in UPF consumption was associated with an increased risk of overall (1.06; 1.03–1.09), ovarian (1.30; 1.13–1.50), and breast (1.16; 1.02–1.32) cancer-related mortality.
Interpretation
Our UK-based cohort study suggests that higher UPF consumption may be linked to an increased burden and mortality for overall and certain site-specific cancers especially ovarian cancer in women.
Funding
The Cancer Research UK and World Cancer Research Fund.
Keywords
Evidence before this study
We searched PubMed using combinations of search terms such as “ultra”, “industrial”, “processed”, “food”, “drink”, and “cancer” on 10 September 2022 with no date or language restrictions. Our search results showed limited prospective evidence for the association between ultra-processed consumption and cancer outcomes. To date, only incidence of the most common cancer sites including colorectal, breast, and prostate cancer has been examined and the cohort studies that assessed total cancer mortality may be potentially limited by small sample size. No previously published cohort study has assessed incidence and mortality for a comprehensive range of site-specific cancers in relation to ultra-processed food consumption, and there is currently no data from the UK despite it is one of the world’s leading consumers of ultra-processed foods.
Added value of this study
Our study provides the first most comprehensive assessment for the prospective associations between ultra-processed food consumption and risk of overall and 34 site-specific cancer incidence and associated mortality. Our findings show that higher consumption of ultra-processed foods is associated with a greater risk of overall cancer and specifically ovarian and brain cancer, as well as increased risk of overall, ovarian, and breast cancer-associated mortality. These associations persisted after adjustment for a range of socio-demographic, smoking status, physical activity, and key dietary factors.
Implications of all the available evidence
Cancer has surpassed cardiovascular disease as the leading cause of premature death in many high-income countries while cancer burden is rising most rapidly in low and middle-income countries. Our study adds important prospective evidence linking ultra-processed food to an increased risk of adverse cancer outcomes. Lowering consumption of ultra-processed foods in dietary patterns may be beneficial for the prevention and risk reduction of overall and certain site-specific cancers.
Introduction
- Ferlay J.
- Laversanne M.
- Ervik M.
- et al.
Cancer is responsible for one in six deaths globally and has surpassed cardiovascular disease as the leading cause of premature mortality in many high-income countries.
- World Health Organization
2020
,
- Bray F.
- Laversanne M.
- Weiderpass E.
- Soerjomataram I.
Cancer. 2021; 127: 3029-3030
However, at least 50% of cancer cases could be potentially preventable and an unhealthy diet is a key modifiable risk factor.
- World Health Organization
2020
,
- World Cancer Research Fund/American Institute for Cancer Research
Continuous Update Project Expert Report, 2018
There are growing concerns over the potential harmful health effects of ultra-processed foods (UPF) – foods that are industrial formulations made by assembling industrially-derived food substances and food additives through a sequence of extensive industrial processes.
- Monteiro C.A.
- Cannon G.
- Levy R.B.
- et al.
Public Health Nutr. 2019; 22: 936-941
UPFs contain little or no whole foods and are often energy dense, high in salt, sugar and fat, low in fibre, and liable to overconsumption.
- Monteiro C.A.
- Cannon G.
- Levy R.B.
- et al.
Public Health Nutr. 2019; 22: 936-941
They are aggressively marketed with strong brands to promote consumption and are gradually displacing traditional dietary patterns based on fresh and minimally processed foods.
- Monteiro C.A.
- Cannon G.
- Levy R.B.
- et al.
Public Health Nutr. 2019; 22: 936-941
- Baker P.
- Machado P.
- Santos T.
- et al.
Obes Rev. 2020; 21e13126
,
- Srour B.
- Kordahi M.C.
- Bonazzi E.
- Deschasaux-Tanguy M.
- Touvier M.
- Chassaing B.
Lancet Gastroenterol Hepatol. 2022; 7: 1128-1140
Evidence has been accumulating on the associations of higher UPF consumption and increased risks of adverse health outcomes including obesity, type 2 diabetes (T2D), and all-cause mortality.
- Srour B.
- Kordahi M.C.
- Bonazzi E.
- Deschasaux-Tanguy M.
- Touvier M.
- Chassaing B.
Lancet Gastroenterol Hepatol. 2022; 7: 1128-1140
However, prospective evidence on the association of UPF consumption and cancer outcomes is limited to a few studies that assessed the incidence of common cancers or total cancer mortality.
- Fiolet T.
- Srour B.
- Sellem L.
- et al.
BMJ. 2018; 360: k322
,
- Wang L.
- Du M.
- Wang K.
BMJ. 2022; 378
,
- Rico-Campà A.
- Martínez-González M.A.
- Alvarez-Alvarez I.
- et al.
BMJ. 2019; 365: 1949
,
- Bonaccio M.
- Di Castelnuovo A.
- Costanzo S.
- et al.
Am J Clin Nutr. 2021; 113: 446-455
,
- Orlich M.J.
- Sabaté J.
- Mashchak A.
- et al.
Am J Clin Nutr. 2022; 115: 1589-1601
Besides their poorer nutritional composition, UPFs may additionally increase cancer risk through neo-formed contaminants during industrial processing, use of some controversial food additives, and certain materials of packaging implicated in exhibiting carcinogenic and/or endocrine-disrupting properties.
- Debras C.
- Chazelas E.
- Srour B.
- et al.
PLoS Med. 2022; 19e1003950
,
- Thoene M.
- Dzika E.
- Gonkowski S.
- Wojtkiewicz J.
Nutrients. 2020; 12: 532
,
- Friedman M.
Food Funct. 2015; 6: 1752-1772
Therefore, this study aims to provide a comprehensive assessment of the association between UPF consumption and risk of overall and 34 site-specific cancer incidence and mortality in a large and contemporary cohort of British adults, in a country with prominently high UPF consumption.
Methods
Data source
- UK Biobank
During recruitment, participants completed questionnaires regarding socio-demographic, lifestyle and psychosocial characteristics, and had objective anthropometrics measurements, medical history and medication use recorded/verified by trained research staff.
- UK Biobank
Dietary intakes were assessed using a web-based, self-administered 24-h recall which was conducted five times between 2009 and 2012. This 24-h recall has been validated against an interviewer-administered 24-h recall showing similar recordings of food items as well as estimated energy and nutrient intakes.
- Liu B.
- Young H.
- Crowe F.L.
- et al.
Public Health Nutr. 2011; 14: 1998-2005
The UK Biobank received ethical approval from the North West Multi-centre Research Ethics Committee (21/NW/0157) and data access was granted by the UK Biobank’s Access Sub-committee.
- UK Biobank
All participants provided written informed consent, allowing for prospective data linkage to health records. The ethical review boards from the International Agency for Research on Cancer (IARC) approved the study.
Dietary exposure and degree of industrial food processing
- Rauber F.
- Chang K.
- Vamos E.P.
- et al.
Eur J Nutr. 2020; 60: 2169-2180
In brief, we applied the NOVA food classification to 24-h recall data assigning each food and beverage item to one of the four main food groups according to their extent and purpose of food processing
- Monteiro C.A.
- Cannon G.
- Levy R.B.
- et al.
Public Health Nutr. 2019; 22: 936-941
: (1) unprocessed or minimally processed foods, e.g. fruit, vegetables, milk and meat; (2) processed culinary ingredients, e.g. sugar, vegetable oils and butter; (3) processed foods, e.g. canned vegetables in brine, freshly made breads and cheeses; and (4) UPFs, e.g. soft drinks, mass-produced industrial-processed breads, sweet or savoury packaged snacks, breakfast ‘cereals’, reconstituted meat products and ready-to-eat/heat foods.
The main exposure variable was individuals’ UPF consumption, expressed as a percentage of UPF content in the total diet (g/day), averaged across multiple 24-h recalls. This weight ratio was preferred over an energy ratio as it better captures UPFs with zero or low-calorie content such as artificially sweetened beverages. We further categorised individuals into UPF consumption quartiles. In sensitivity analysis, we computed UPF consumption as a percentage of total energy intake (kcal/day) for comparison.
Outcome measures
- UK Biobank
Cancer registries were coded in the 10th revision of the International Classification of Diseases (ICD-10) and the third edition of ICD for Oncology (ICD-O-3) morphology codes where appropriate, and were available up to 31 July 2019 for England and Wales, and 31 October 2015 for Scotland. Mortality registries were coded using ICD-10 and available up to 31 January 2021. Cancer deaths were defined as primary/underlying cause of death. We examined all cancers (C00–C97, except for non-melanoma skin cancer C44) and 34 site-specific cancers. Detailed list, coding and case numbers for each site-specific cancer are presented in Appendix Table S1 for cancer incidence and Appendix Table S2 for cancer mortality. Cancers with small case numbers (n
Study covariates
- UK Biobank
Additional covariates considered in sensitivity analysis included intake of sodium, total fat, carbohydrate, red meat, processed meat, fibre, and calcium; and presence of diabetes, cardiovascular disease (angina, myocardial infarction, and stroke), depression, and hypertension at baseline. Missing data were under 3% except for physical activity (15.1% missing) and average household income (9.4% missing). We used multiple imputation by chained equation with 10 imputed datasets to estimate missing covariate data under assumption of missing at random and the analytical results were combined using Rubin’s rule.
Statistical analysis
The study cohort included 197,426 UK Biobank participants with 24-h recall data (78,330 [39.7%], 45,137 [22.9%], 39,841 [20.2%], 28,654 [14.5%], and 5464 [2.8%] with one to five recalls, respectively) after excluding 12,680 individuals with pre-existing cancers, 173 individuals who were pregnant/unsure, and 668 individuals with an implausible total daily energy intake outside of 500–5000 kcal/day (Appendix Fig. S1). We compared participants’ baseline characteristics by quartile of UPF consumption using analysis of variance for continuous and χ2 test for categorical variables. Furthermore, we estimated and graphically presented the mean percentage of daily food intake (or daily energy intake) for each NOVA subgroup.
We used Cox proportional hazards regression with age as the underlying time metric to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between UPF consumption and each cancer outcome. Participants contributed person time until the date of cancer incidence/mortality, death of non-cancer causes, lost to follow-up, or end of study period, whichever occurred first. We built four models in incremental steps: Model 1 included age (timescale) and UPF consumption, stratified by sex; Model 2 additionally included ethnicity, smoking status, physical activity, average household income, highest educational attainment, alcohol intake, and additionally stratified by height, family history of cancer, IMD, and geographical region; Model 3 additionally included BMI category; Model 4 (final model) additionally included daily energy intake. For breast, uterus, and ovarian cancer outcomes, we additionally included in Model 2: baseline menopausal status, use of oral contraceptives, use of hormone replacement therapy, and parity. We performed separate Cox regression for each measurement of UPF consumption: continuous per 10% absolute increment in UPF content, categorical UPF quartiles, and trend using ordinal UPF quartiles. The proportional hazards assumption was evaluated by tests based on Schoenfeld residuals. Potential non-linearity in the association were examined by considering higher order polynomials of UPF consumption and restricted cubic spline function but none were identified. Lung cancer outcomes were additionally stratified by smoking status, and head and neck cancer incidence was stratified by smoking status and alcohol consumption in an exploratory analysis.
The following sensitivity analyses were performed based on Model 4 (final model): (i) additionally adjusting for key dietary factors including sodium, total fat, and carbohydrate intake while total energy intake was removed due to high correlation (>0.74) between total energy intake and these nutrients. Colorectal cancer analyses were additionally adjusted for red meat, processed meat, fibre, and calcium intake; (ii) additionally adjusting for intake of sodium, trans fat, and free sugars while total energy intake was removed from the model (results were consistent when intake of saturated fat was adjusted for instead of trans fat but they were not simultaneously included in the model due to a high correlation of 0.79); (iii) additionally adjusting for fruit and vegetable intake; (iv) removing (ultra-processed) alcohol intake from the UPF exposure and also deducting the energy contribution of alcohol consumption from the total energy intake; (v) additionally adjusting for baseline presence of diabetes, CVD, depression, and hypertension; (vi) additionally adjusting for number of 24-h recalls; (vii) excluding participants with follow-up time <2 years.
All statistical analyses were performed using Stata SE version 12.1. All tests were two-sided and a P < 0.05 was considered statistically significant. A more conservative Bonferroni-corrected P < 0.05 accounting for multiple comparisons (computed by multiplying the original P-value by 45 for analysis of cancer incidence and 28 for analysis of cancer mortality) is additionally presented.
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation of study findings, writing of the report, or decision to submit the paper for publication. All authors had full access to all the data in the study, approved the final manuscript, and accept responsibility for the decision to submit for publication.
Results
Table 1Baseline characteristics by quartile of ultra-processed food consumption among UK Biobank participants.
UPF, ultra-processed food; SD, standard deviation.
a UPF consumption was defined as the percentage of its weight contribution relative to total food intake measured in g/day. Study participants were further categorized into quartiles (Q1-Q4 represents lowest to highest quartile of UPF consumption).
b χ2 tests (for categorical variables) and ANOVA tests (for continuous variables) were used to compare cohort characteristics across UPF consumption quartiles.
c Additional characteristics among female participants (n = 107919).
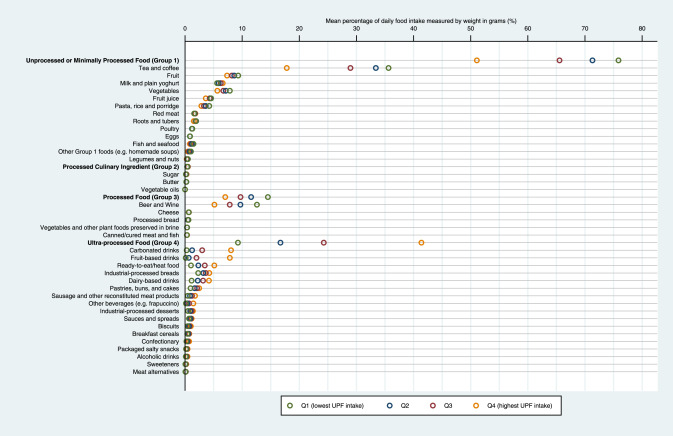
Fig. 1Sources of NOVA subgroups in the total diet by quartile of ultra-processed food consumption. UPF, ultra-processed food. UPF consumption was defined as the percentage of its weight contribution relative to total food intake measured in g/day. Study participants were further categorised into quartiles (Q1-Q4 represents lowest to highest quartile of UPF consumption).
UPF consumption and cancer incidence
Table 2Association between ultra-processed food consumption in the total diet and cancer incidence.
UPF, ultra-processed food; HR, hazard ratio; CI, confidence interval; Ref, reference category; CLL, Chronic lymphocytic leukemia; SLL, Small lymphocytic lymphoma.
All models were fully adjusted with age (underlying timescale), ethnicity, smoking status, physical activity level, average household income, highest educational attainment, alcohol intake, body mass index, total daily energy intake, and stratified by sex, height, family history of cancer, index of multiple deprivation quintile, and geographical region. Analyses of female-specific cancers were additionally adjusted for baseline menopausal status, use of oral contraceptives, use of hormone replacement therapy, and parity.
a UPF consumption was defined as the percentage of its weight contribution relative to total food intake measured in g/day. Study participants were further categorized into quartiles (Q1-Q4 represents lowest to highest quartile of UPF consumption).
b Modelling for breast, uterus and ovarian cancers were conducted in women only (n = 107919), modelling for prostate cancer were conducted in men only (n = 89507).
c P < 0.05.
d P < 0.01.
e P < 0.001.
f Bonferroni-corrected P < 0.05.
Furthermore, when analysing UPF consumption quartiles (Table 2), participants with the highest compared with lowest UPF quartile had a higher risk of overall cancer by 7% (95% CI, 1.02–1.14), lung cancer by 25% (95% CI, 1.01–1.57), brain cancer by 52% (95% CI, 1.04–2.23), and diffuse large B-cell lymphoma by 63% (95% CI, 1.00–2.66). Conversely, a significantly lower risk of head and neck cancer was observed among those with higher UPF quartile (e.g. HR, 0.59; 95% CI, 0.41–0.85 for the highest compared with lowest UPF quartile). Similarly, stratified analyses showed lower risk patterns for head and neck cancer among never smokers, ex-smokers and all alcohol consumption groups, but most findings did not reach statistical significance (Appendix Table S4).
UPF consumption and cancer mortality
Table 3Association between ultra-processed food consumption in the total diet and cancer-related mortality.
UPF, ultra-processed food; HR, hazard ratio; CI, confidence interval; Ref, reference category.
All models were fully adjusted with age (underlying timescale), ethnicity, smoking status, physical activity level, average household income, highest educational attainment, alcohol intake, body mass index, total daily energy intake, and stratified by sex, height, family history of cancer, index of multiple deprivation quintile, and geographical region. Analyses of female-specific cancers were additionally adjusted for baseline menopausal status, use of oral contraceptives, use of hormone replacement therapy, and parity.
a UPF consumption was defined as the percentage of its weight contribution relative to total food intake measured in g/day. Study participants were further categorized into quartiles (Q1-Q4 represents lowest to highest quartile of UPF consumption).
b Modelling for breast, uterus and ovarian cancers were conducted in women only (n = 107919), modelling for prostate cancer were conducted in men only (n = 89507).
c P < 0.05.
d P < 0.01.
e P < 0.001.
f Bonferroni-corrected P < 0.05.
Calorie contribution of UPFs in diet and cancer risk
The mean calorie contribution of UPFs was 48.6% (SD, 15.8%) of total energy intake (kcal/day) and ranged from 28.4% (7.6%) to 68.7% (7.5%) for the lowest to highest UPF quartiles (Appendix Fig. S4). The fully adjusted results suggest a positive association between the proportion of daily calories sourced from UPFs and incidence of overall cancer, cancers of lymphatic and haematopoietic tissues, and ovarian cancer (Appendix Table S6). Evaluation of cancer mortality showed significantly positive associations for overall, oesophagus, and ovarian cancers (Appendix Table S7).
Discussion
This large prospective cohort analysis conducted within the UK Biobank provides a comprehensive assessment of associations between UPF consumption and risk of many site-specific cancer outcomes for the first time to our knowledge. There are three particularly noteworthy findings: First, every 10% increment in UPF content of diet was associated with an increased incidence of overall cancer by 2% and ovarian cancer by 19%. Second, participants with the highest compared with lowest UPF consumption quartile had higher incidence of overall and brain cancer, and a lower incidence of head and neck cancer. Finally, every 10% increment in UPF consumption was associated with increased mortality of overall cancer by 6%, breast cancer by 16%, and ovarian cancer by 30%. These associations persisted after adjustment for a range of key socio-economic, behavioural and dietary factors.
- Fiolet T.
- Srour B.
- Sellem L.
- et al.
BMJ. 2018; 360: k322
The French NutriNet-Santé study showed a 12% and 11% increase in risk of overall and breast cancer per 10% increment in UPF consumption (g/day), and no evidence of association for prostate and colorectal cancer.
- Fiolet T.
- Srour B.
- Sellem L.
- et al.
BMJ. 2018; 360: k322
A recent study from the US found a 29% increase in the risk of colorectal cancer among men in the highest quintile of UPF consumers compared with the lowest (based on servings/day).
- Wang L.
- Du M.
- Wang K.
BMJ. 2022; 378
Our findings are more aligned with the NutriNet-Santé study for overall, prostate, and colorectal cancer incidence, but not for breast cancer incidence. While we used the same measure of UPF consumption (weight ratio) as the NutriNet-Santé study, the US study had a much longer follow-up time (24–28 years compared with 5 years in the NutriNet-Santé and 10 years in this study). Moreover, our study cohort had a larger proportion of never smokers than in previous studies and these differences in characteristics, design and setting may all contribute to the differences in findings. Mortality of site-specific cancers have not been previously assessed, but overall cancer mortality has been examined in three cohort studies conducted in Spain, Italy, and North America with similar lengths of follow-up to this study.
- Rico-Campà A.
- Martínez-González M.A.
- Alvarez-Alvarez I.
- et al.
BMJ. 2019; 365: 1949
,
- Bonaccio M.
- Di Castelnuovo A.
- Costanzo S.
- et al.
Am J Clin Nutr. 2021; 113: 446-455
,
- Orlich M.J.
- Sabaté J.
- Mashchak A.
- et al.
Am J Clin Nutr. 2022; 115: 1589-1601
None of the three studies, however, have identified any significant association with cancer mortality. Our study cohort is more than two-folds larger than previous studies and comparatively older due to the recruitment of middle-aged adults. Moreover, our study cohort was originated from a population with a substantially greater consumption of UPFs. Importantly, our study presents findings for many less common cancers not examined before, and our findings of the positive associations between UPF consumption and risks and associated mortality of overall and ovarian cancer were consistent among weight and energy ratio measures of UPF consumption.
- World Health Organization
2020
,
- World Cancer Research Fund/American Institute for Cancer Research
Continuous Update Project Expert Report, 2018
However, dietary patterns with a high UPF content are generally nutritionally inferior and are higher in energy, total and saturated fats, salt, and free sugars, and lower in fibre and several micronutrients.
- Monteiro C.A.
- Cannon G.
- Levy R.B.
- et al.
Public Health Nutr. 2019; 22: 936-941
Alteration of food matrices by ultra-processing results in degradation of food health potential and deterioration of nutrient bioavailability and bioaccessibility.
- Fardet A.
- Rock E.
Eur J Nutr. 2022; 61: 2239-2253
Furthermore, evidence has been accumulating on the strong obesity and T2D-promoting potential of UPFs,
- Srour B.
- Kordahi M.C.
- Bonazzi E.
- Deschasaux-Tanguy M.
- Touvier M.
- Chassaing B.
Lancet Gastroenterol Hepatol. 2022; 7: 1128-1140
both of which are risk factors for many cancers including those of the digestive tract and some hormone-related cancers in women.
- World Cancer Research Fund/American Institute for Cancer Research
Continuous Update Project Expert Report, 2018
,
- Avgerinos K.I.
- Spyrou N.
- Mantzoros C.S.
- Dalamaga M.
Metabolism. 2019; 92: 121-135
,
- Pearson-Stuttard J.
- Papadimitriou N.
- Markozannes G.
- et al.
Cancer Epidemiol Biomarkers Prev. 2021; 30: 1218-1228
- Debras C.
- Chazelas E.
- Srour B.
- et al.
PLoS Med. 2022; 19e1003950
while higher intake of nitrate and nitrite from food additives was associated with increased risk of breast and prostate cancer, respectively.
- Chazelas E.
- Pierre F.
- Druesne-Pecollo N.
- et al.
Int J Epidemiol. 2022; 51: 1106-1119
Higher dietary exposure of acrylamide, an industrial chemical formed during high-temperature cooking procedures, was found associated with an increased risk of ovarian and endometrial cancers.
- Adani G.
- Filippini T.
- Wise L.A.
- Halldorsson T.I.
- Blaha L.
- Vinceti M.
Cancer Epidemiol Biomarkers Prev. 2020; 29: 1095-1106
Phthalates and bisphenols are endocrine-disrupting chemicals commonly found in food storage, packaging and contacting materials, and higher urinary concentration of phthalates and bisphenols-F (analogue of the more regulated bisphenol-A) have been detected in individuals with higher UPF consumption.
- Martínez Steele E.
- Khandpur N.
- da Costa Louzada M.L.
- Monteiro C.A.
PLoS One. 2020; 15e0236738
Available data on bisphenols are predominantly experimental but have consistently shown many toxic effects including for human breast cancer and damage to DNA, nervous and immune systems.
- Thoene M.
- Dzika E.
- Gonkowski S.
- Wojtkiewicz J.
Nutrients. 2020; 12: 532
Epidemiological studies have suggested a positive association between phthalates and T2D and insulin resistance.
- Radke E.G.
- Galizia A.
- Thayer K.A.
- Cooper G.S.
Environ Int. 2019; 132104768
,
- Shoshtari-Yeganeh B.
- Zarean M.
- Mansourian M.
- et al.
Environ Sci Pollut Res Int. 2019; 26: 9435-9442
Recent animal models have shown that phthalates may induce neuroinflammation and disruption of the blood–brain barrier.
- Ahmadpour D.
- Mhaouty-Kodja S.
- Grange-Messent V.
Chemosphere. 2021; 282131013
No previous studies have assessed the link between UPF consumption and brain cancer, but human studies have demonstrated associations with potential harmful effects on brain functions.
- Li H.
- Li S.
- Yang H.
- et al.
Neurology. 2022; 10WNL.0000000000200871
,
- Hall K.D.
- Ayuketah A.
- Brychta R.
- et al.
Cell Metab. 2019; 30: 67-77.e3
- Liu B.
- Young H.
- Crowe F.L.
- et al.
Public Health Nutr. 2011; 14: 1998-2005
- Rauber F.
- Steele E.M.
- da Costa Louzada M.L.
- Millett C.
- Monteiro C.A.
- Levy R.B.
PLoS One. 2020; 15e0232676
However, this study has reported important associations comparing cancer risk and mortality by levels of UPF consumption which may still be generalisable to the wider population or similar cohorts in other contexts. Second, misclassification of a few food items may occur owing to limited food processing information and we assigned them to the most probable food group based on published findings of common foods and drinks consumed in the UK.
- Rauber F.
- Steele E.M.
- da Costa Louzada M.L.
- Millett C.
- Monteiro C.A.
- Levy R.B.
PLoS One. 2020; 15e0232676
Third, while we considered the average of multiple 24-h recalls the best representation of usual dietary intake, 39.7% of the cohort had only one 24-h recall and may be prone to measurement error owing to its limited ability to fully capture individuals’ variation in diet. Fourth, the associations for head and neck cancer incidence may be partly due to the complex interrelationships between UPF consumption, alcohol intake and smoking. However, we could not explore these further due to limited sample size shown in exploratory analysis. Finally, although we adjusted the analyses for a wide range of potential confounders including key lifestyle and nutritional factors, residual confounding may have affected the findings due to observational nature of the study.
In summary, this large contemporary prospective study of middle-aged UK adults found that higher UPF consumption was associated with a greater incidence and mortality of overall and certain site-specific cancers. Although causality may not be implied owing to the observational nature of the study, these findings highlight the importance of considering degrees of food processing in diets. In particular, the associations were found most consistent for overall cancer and ovarian cancer outcomes in women. These findings suggest that limiting UPF consumption may be beneficial to prevent and reduce the modifiable burdens of cancer.
Contributors
KC, CM, and EPV conceptualised the study and all authors contributed to the study design. KC compiled the data and performed statistical analyses with supervisory input from EPV. KC and EPV had access and verified the underlying data used in this study. All authors contributed to the finalisation of statistical models and interpretation of findings. KC and EPV wrote the first draft of the manuscript, and MJG, FR, RBL, IH, NK, and CM critically reviewed and edited the manuscript. All authors had full access to all the data in the study, approved the final manuscript, and accept responsibility for the decision to submit for publication.
Data sharing statement
UK Biobank data are available through application to the database https://www.ukbiobank.ac.uk/.
Declaration of interests
All authors declare no conflict of interest.
Acknowledgements
This work was supported by Cancer Research UK C33493/A29678. Funding IIG_FULL_2020_033 was obtained from World Cancer Research Fund (WCRF UK), as part of the World Cancer Research Fund International grant programme. Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization. FR declares funding from The São Paulo Research Foundation (FAPESP) 16/14302-7 and 18/19820-1. This research has been conducted using the UK Biobank Resource under Application Number 29239. The authors would like to thank the participants of the UK Biobank study.
Appendix A. Supplementary data
References
- 1.
Global cancer observatory: cancer tomorrow.
International Agency for Research on Cancer, Lyon, France2020 (Available from:)
(Last accessed: 27/Jun/2022)
- 2.
WHO report on cancer: setting priorities, investing wisely and providing care for all.
2020
- 3.
The ever-increasing importance of cancer as a leading cause of premature death worldwide.
Cancer. 2021; 127: 3029-3030
- 4.
Diet, nutrition, physical activity and cancer: a global perspective.
Continuous Update Project Expert Report, 2018
- 5.
Ultra-processed foods: what they are and how to identify them.
Public Health Nutr. 2019; 22: 936-941
- 6.
Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers.
Obes Rev. 2020; 21e13126
- 7.
Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights.
Lancet Gastroenterol Hepatol. 2022; 7: 1128-1140
- 8.
Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort.
BMJ. 2018; 360: k322
- 9.
Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohorts.
BMJ. 2022; 378
- 10.
Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study.
BMJ. 2019; 365: 1949
- 11.
Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study.
Am J Clin Nutr. 2021; 113: 446-455
- 12.
Ultra-processed food intake and animal-based food intake and mortality in the Adventist Health Study-2.
Am J Clin Nutr. 2022; 115: 1589-1601
- 13.
Artificial sweeteners and cancer risk: results from the NutriNet-Santé population-based cohort study.
PLoS Med. 2022; 19e1003950
- 14.
Bisphenol S in food causes hormonal and obesogenic effects comparable to or worse than bisphenol A: a literature review.
Nutrients. 2020; 12: 532
- 15.
Acrylamide: inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans.
Food Funct. 2015; 6: 1752-1772
- 16.
UK Biobank.
(Last accessed: 31/Aug/2022)
https://www.ukbiobank.ac.uk/Date: 2022
- 17.
Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies.
Public Health Nutr. 2011; 14: 1998-2005
- 18.
Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank.
Eur J Nutr. 2020; 60: 2169-2180
- 19.
Chronic diseases are first associated with the degradation and artificialization of food matrices rather than with food composition: calorie quality matters more than calorie quantity.
Eur J Nutr. 2022; 61: 2239-2253
- 20.
Obesity and cancer risk: emerging biological mechanisms and perspectives.
Metabolism. 2019; 92: 121-135
- 21.
Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies.
Cancer Epidemiol Biomarkers Prev. 2021; 30: 1218-1228
- 22.
Nitrites and nitrates from food additives and natural sources and cancer risk: results from the NutriNet-Santé cohort.
Int J Epidemiol. 2022; 51: 1106-1119
- 23.
Dietary intake of acrylamide and risk of breast, endometrial, and ovarian cancers: a systematic review and dose–response meta-analysis.
Cancer Epidemiol Biomarkers Prev. 2020; 29: 1095-1106
- 24.
Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older.
PLoS One. 2020; 15e0236738
- 25.
Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence.
Environ Int. 2019; 132104768
- 26.
Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance.
Environ Sci Pollut Res Int. 2019; 26: 9435-9442
- 27.
Disruption of the blood-brain barrier and its close environment following adult exposure to low doses of di (2-ethylhexyl) phthalate alone or in an environmental phthalate mixture in male mice.
Chemosphere. 2021; 282131013
- 28.
Association of ultraprocessed food consumption with risk of dementia: a prospective cohort.
Neurology. 2022; 10WNL.0000000000200871
- 29.
Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake.
Cell Metab. 2019; 30: 67-77.e3
- 30.
Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008-2016).
PLoS One. 2020; 15e0232676
Article info
Publication history
Published: January 31, 2023
Accepted: January 10, 2023
Received in revised form: January 8, 2023
Received: October 26, 2022
Identification
Copyright
© 2023 The Author(s). Published by Elsevier Ltd.
User license
Creative Commons Attribution (CC BY 4.0) |
Creative Commons Attribution (CC BY 4.0)
Permitted
- Read, print & download
- Redistribute or republish the final article
- Text & data mine
- Translate the article
- Reuse portions or extracts from the article in other works
- Sell or re-use for commercial purposes
