You may be familiar with the concept of food contamination caused by chemicals from food packaging. But did you know that these same chemicals can enter the food chain well before that burger has even been made, let alone cooked or served? The dangers of per- and polyfluoroalkyl substances (PFAS) have been repeatedly highlighted in recent years, but detecting their presence, let alone preventing the many routes of ingress into our food chain, is a challenge.
We spoke to Dr Katherine C. Hyland, Applications Specialist, Environmental, Food and Beverage at SCIEX, about the many routes by which PFAS enter the food chain, the problems they cause and why they can be such a headache for analysts.
Karen Steward (KS): How do PFAS get into our food and why are they such a problem?
Katherine Hyland (KH): There are a few major routes by which PFAS can enter our foods and food supply. The three which have been most concerning and most studied to date include:
· Leaching from treated food packaging materials
· Bioaccumulation in food organisms high in the trophic chain
· Accumulation in produce treated with contaminated water or biosolids-amended soils
Of these, it seems that leaching from food packaging has received the most significant media attention and studied.1 PFAS are used in food packaging that needs to resist saturation by oils and water (such as steam). Common examples include paper wrappers like those for burgers and sandwiches, paperboards like pizza boxes or french fry holders, microwave popcorn bags, paper cups and more.
What’s noteworthy about PFAS in food contact materials (FCMs) is that they encompass a range of chemical classes. Besides the common C8 carboxylate and sulfonate species perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), chemicals used for FCMs include fluorotelomer alcohols (FTOHs), polyfluoroalkyl phosphate esters (PAPs), as well as other chain lengths of the carboxylates and sulfonates.2
So, when we consider entry of PFAS by accidental consumption due to food contact, we actually must think about a wider range of chemical constituents than is typically considered for environmental samples. For instance, Europe adopted the Commission Recommendation 2010/161/EU to monitor PFAS in food but it is primarily on the commonly known PFAS—PFOS and PFOA.3 Similarly, the established PFAS health advisory guideline in the United States, EPA Method 537.1, is focused on water and only mentions PFOS and PFOA.4 That said, the United States Food and Drug Administration (FDA) recently developed studies to quantify PFAS in food.5 Nevertheless, substances such as FTOHs and PAPs are absent from these guidelines.
Bioaccumulation to higher trophic levels has been primarily observed/studied in marine systems. The cycle usually resembles PFAS entering water systems, contaminating low-level organisms like amphipods. These organisms are eaten by smaller fish, which are then consumed by larger fish and mammals. Studies have found positive correlations between the serum concentrations of long-chain PFAS in people, and the seafood consumption of the community.6,7 This is particularly interesting as while “legacy” contaminants like PCBs and PBDEs also bioaccumulate up the food chain, the mechanisms by which those happen are different from PFAS. Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs), for example, accumulate in fatty tissues. While the accumulation behavior of long-chain PFAS mimics that of these highly nonpolar legacy species, PFAS does not accumulate in fatty tissues. Their unique chemical properties make it more difficult to predict the partitioning behavior of PFAS in animal organisms.
Fruits and vegetables can also bioaccumulate PFAS when they are irrigated with PFAS-contaminated water. While this may seem intuitive, there is a plot twist—it is the shorter chain PFAS that are preferentially taken up by food crops from contaminated water.
This is important from two main perspectives. First, it is counter to the modeled accumulation of PFAS in animal organisms, which preferentially accumulate the longer-chain species. And second, because it means that the persistence of short-chain PFAS through water and wastewater treatment processes puts commercial food crops grown with treated water at risk for containing PFAS chemicals when sold for human consumption.8
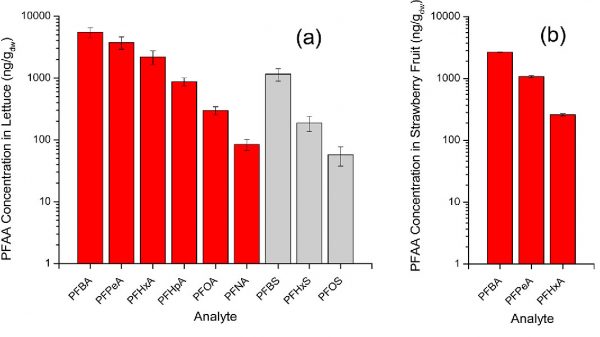
The figure shows concentrations of PFAAs in lettuce leaves (a) and strawberry fruit (b) for the aqueous applied PFAA concentration of 10 μg/L. Reprinted (adapted) with permission from Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water. Credit: Blaine, Andrea C. et al. Environ. Sci. Technol., (2014): 48, 24, 14361-8, doi: 10.1021/es504150h. Copyright (2020) “American Chemical Society”.
Lastly, the uptake of PFAS from biosolids-amended soil into food crops has been identified as another potential pathway for entry into the human food supply. It has been common practice for decades to use the solid sludge material from biological wastewater treatment as a carbon-rich soil amendment for American farms. However, the physicochemical properties of PFAS lend a tendency to sorb to the solid sludge phase during treatment. This means the sorbed PFAS can be carried from the wastewater treatment plant directly to the soils for growing a variety of crops.9
As we can see, the routes in which humans might consume PFAS in their food products are diverse. These include packaging contact, accumulation up through animal food chains, or exposure of produce during farming with contaminated water or soil. And these only represent human exposure through diet. We also know PFAS in drinking water is a potential problem, as well as in various other commercially available items such as waterproofing agents. With all these potential sources of exposure, it can be challenging to ascertain which products or practices represent a genuine risk to human health.
KS: Are some people more at risk from ingesting PFAS and are there any steps they can take to minimize their PFAS intake?
KH: Populations that consume seafood primarily in their diets appear to have higher levels of PFAS in their bloodstreams.6,7 Some freshwater locations in the U.S. have been found to contain measurable PFAS contamination and have prompted local advisories against fishing or consuming fish from the contaminated areas.10 But, as we see above, there are many potential routes for PFAS entry into foods, making it challenging to predict the particular route responsible for human exposure.
KS: How do you think the boom in convenience and takeaway foods has impacted our exposure to PFAS?
KH: Food packaging for takeaway foods is not a new product, and even the use of PFAS as a functional treatment for these products is not new. It is only in the past decade that there has been a growing awareness of the potential for human risk due to PFAS consumption. With so many routes for human exposure and consumption of PFAS, a question arises: what proportion of PFAS in humans has come from dietary exposure? And of that, what proportion might be from food contact materials? And of course, the degree of volume and frequency of takeaway foods in an individual’s diet can be highly variable between individuals, cultures or geographic localities. All of these make it difficult to pinpoint the degree to which takeaway food packaging has driven the observed occurrence of PFAS in human populations. That said, there have been a few reports suggesting based on modeled data that the bulk of PFAS measured in humans might come from dietary consumption.
KS: What are the biggest challenges in detecting PFAS?
KH: Background levels due to ubiquity represent a major analytical obstacle. What this means is, that the presence of PFAS is so universal in products in our environment, that being able to measure the low levels that are relevant becomes challenging. Sample contamination during preparation and analysis or originating from analytical instrumentation can vastly impact the ability to achieve sensitive and accurate quantification.
Recognizing this, several analytical procedures have become a common practice for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of PFAS. For instance, all Teflon components of the LC system are typically swapped with alternative stainless-steel components to avoid leaching of PFAS from the Teflon. There is also the addition of a small delay column upstream of the autosampler, which will retard any PFAS coming from the LC system away from the analytical peak, allowing the peak to be consistently and accurately integrated during quantitative processing.
KS: How are food and packaging companies looking to address the presence of PFAS in foods?
KH: Indeed, the landscape is always changing as companies and regulators adjust manufacturing practices and requirements. Chemours (formally DuPont) have been reported to be pulling back on some PFAS chemicals in food packaging11 and a global decline in PFOS and PFOA production has led to a decrease in observed concentrations of these species in waters and biota.12 With that, however, has been a reported increase in the observed concentrations of those PFAS compounds manufactured as PFOS and PFOA replacements.
KS: Do you feel that there are any additional steps that regulatory authorities could be taking to protect the public from PFAS hazards? Are there any significant gaps in knowledge that could contribute to this?
KH: The risks posed by PFOS and PFOA replacement compounds are still largely unknown. With increased awareness and regulation, these and other “legacy” PFAS compounds have been gradually phased out of production and manufacturing. This is reflected in the occurrence data measured in environmental and biota samples over the past few years. It is also reported that there has been a decrease in the occurrence of PFOS and PFOA. Still, the industry has swiftly replaced or moved to replace these with alternative PFAS compounds, including the so-called “Gen X” compounds. The long list of individual PFAS compounds and varied chemical properties make it very difficult to predict or study the toxicological risk of each individual PFAS compound. This represents a vital knowledge gap that regulatory bodies must be cognizant of when applying regulations to the use of PFAS compounds.
References
1. https://www.technologynetworks.com/applied-sciences/news/how-many-chemical-nasties-are-in-your-take-away-324995.
2. Fluorinated Compounds in U.S. Fast Food Packaging. Laurel A. Schaider, Simona A. Balan, Arlene Blum, David Q. Andrews, Mark J. Strynar, Margaret E. Dickinson, David M. Lunderberg, Johnsie R. Lang, and Graham F. Peaslee. Environ. Sci. Technol. Lett., (2017): 4, 3, 105-111, DOI: 10.1021/acs.estlett.6b00435.
3. Commission Recommendation of 17 March 2010 on the monitoring of perfluoroalkylated substances in food, retrieved Feb 20, 2020 from https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:068:0022:0023:EN:PDF.
4. Shoemaker, J. AND Dan Tettenhorst. Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington, DC, 2018.
5. “U.S. Food and Drug Administration.” U.S. Food and Drug Administration , 31 Oct. 2019, https://www.fda.gov/food/cfsan-constituent-updates/fda-makes-available-testing-method-pfas-foods-and-final-results-recent-surveys.
6. Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P, Sunderland EM. Environ. Sci. Technol., (2018): 52, 6, 3738–3747, doi:10.1021/acs.est.7b06044.
7. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Marianne Hauka, Urs Berger, Haakon Hop, Bjørn Gulliksen, Geir W. Gabrielsen Environ. Pollut., (2007): 148, 1, 360-71, DOI: 10.1016/j.envpol.2006.09.021.
8. Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water. Blaine, Andrea C. et al. Environ. Sci. Technol., (2014): 48, 24, 14361-8, doi: 10.1021/es504150h.
9. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids-amended soils. Blaine, Andrea C. et al. Environ. Sci. Technol., (2014): 48, 14, 7858-65, doi: 10.1021/es500016s.
10. Goodrow, Sandra M, et al. “Investigation of Levels of Perfluorinated Compounds in New Jersey Fish, Surface Water, and Sediment .” Division of Science and Research Research Project Summary, Sept. 2019, https://www.nj.gov/dep/dsr/publications/Investigation_of_Levels_of_Perfluorinated_Compounds_in_New_Jersey_Fish_Surface_Water_and_Sediment_RPS.pdf.
12. Shifting Global Exposures to Poly- and Perfluoroalkyl Substances (PFASs) Evident in Longitudinal Birth Cohorts from a Seafood-Consuming Population. Clifton Dassuncao, Xindi C. Hu, Flemming Nielsen, Pál Weihe, Philippe Grandjean, and Elsie M. Sunderland. Environ. Sci. Technol., (2018): 52 (6), 3738-3747. DOI: 10.1021/acs.est.7b06044.
Dr. Katherine Hyland was speaking to Dr. Karen Steward, Science Writer for Technology Networks.