It’s not your imagination: gin and tonic water actually taste better together than apart. The duo is greater than the sum of their parts thanks to their chemical makeup; your nose, mouth, and brain are wired to light up when they encounter the cocktail. Now, if only food scientists could figure out exactly why.
“One of the reasons I love talking about food chemistry and the gin and tonic problem in particular is that we don’t know,” Matthew Hartings, a faculty member in the department of chemistry at American University who has put a lot of thought into the mystery of the delicious G&T, shared. “We have some ideas, but a full account of it, we don’t know.”
Let’s start with what we do know. What we taste, and more importantly what we smell, arises from molecules inside the drink. In the case of a G&T, these molecules come from botanicals — primarily juniper — infused into the gin (which is drawn out by ethanol during distillation) and from quinine in the tonic, which gives the mixer its unique bitter taste. These molecules are delivered to our mouths by drinking or to our noses, where most of our flavor receptors actually are, by evaporation. While ice adds a cool crispness to the taste, it also dampens the molecular activity. This is why extra bubbly tonic helps to deliver more flavor — by transporting the chemicals up the liquid and into our mouths.
The next piece of the puzzle is how we taste. Molecules in your drink fit into protein receptors in the nose and mouth, triggering signals that go to your brain and giving you a sense of taste and smell.
But we’re not simply talking about individual molecules in the case of gin and tonic. We’re talking about aggregate molecules, which combine individual chemicals into new molecules that taste completely different. Unlike oil and water, which separate violently, the molecules in gin and tonic water naturally attract one another.
“When we start talking about how molecules are attracted to one another,” Hartings explained, “the general rule of thumb is if two molecules look like one another, and they have the same patterns of carbons and hydrogens and oxygens, and they have the same backbones and substance, they’re going to be attracted to one another.” If you want to get into the scientific weeds about it, similar chemical structures generate electric dipoles that attract one another. If you’d like the Cocktail Science for Dummies version, just take a look at these graphics Hartings generated (highlighted to show similar chemical structures).
Gin botanicals:
Recommended Video
Drink
The History of Four Loko
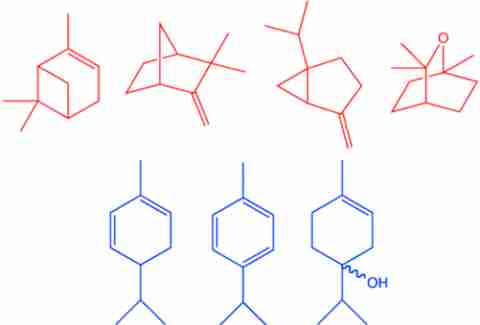
Quinine:
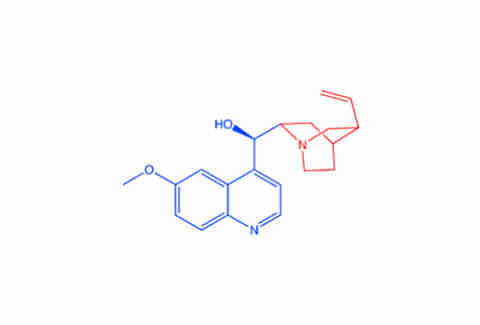
Molecules in gin and tonic water naturally attract and form aggregates, and these aggregates — along with some individual molecules — float up into the receptors within your nose and mouth. From here, things can get a bit more complicated.
Gin molecules can fit into certain proteins while tonic molecules can fit into some of these same proteins. The same goes for the aggregate molecules, which can fit into some receptors that work with each ingredient and also some new proteins. All of these interactions send different signals to the brain, but size and shape aren’t the only things that matter. “How long these molecules are in that flavor receptor and how tightly they bind all affect the signal that gets sent to your brain,” Hartings said. Plus, it’s not as if the molecules are lining up politely to take turns sending different signals. “It’s a battle royale — these molecules are duking it out to see which one will go into this flavor receptor.”
The sheer complexity explains why food scientists still can’t figure out why gin and tonic taste especially good together. “You’re thinking about hundreds or thousands of different molecules in a glass and then the several hundred different kinds of proteins in your nose collecting all those interesting flavors,” Hartings said. “And you have to think about how all those molecules interact with one another, how they interact individually with those proteins, and how those proteins interact on a whole with all those molecules at once. It’s a big messy problem.”
Compound this challenge with the addition of sugar in the tonic water and lime juice in the garnish, not to mention the various combinations of botanicals that gin distillers can use, and you have a recipe for an incredibly difficult scientific quandary. You also have the recipe for a darn simple drink that’s utterly, mysteriously delicious.
Sign up here for our daily Thrillist email, get Eatmail for more food coverage, and subscribe here for our YouTube channel to get your fix of the best in food/drink/fun.
