
Credit: Ali Criscitiello
Researchers collected these ice cores to study levels of CFC replacements in the Arctic.
Most countries began phasing out ozone-depleting chlorofluorocarbons (CFCs) in the early 1990s under the Montreal Protocol on Substances that Deplete the Ozone Layer. Replacing these refrigerants and propellants did the trick—the ozone layer recovered.
However, a new analysis of Arctic ice cores points to an unintended consequence of this regulation. Over the years, the compounds that replaced CFCs have been transported and transformed in the atmosphere, depositing far from their sources. As a result, short-chain fluorinated alkyl acids, which are highly mobile persistent organic pollutants, are accumulating in the Arctic, and likely all around the Northern Hemisphere (Geophys. Res. Lett. 2020, DOI: 10.1029/2020GL087535).
York University atmospheric chemist Cora Young has previously found long-chain per- and polyfluoroalkyl substances (PFAS) in Arctic ice. In her new research, she focused on smaller compounds in this class, which haven’t been studied as much. Researchers had hypothesized that levels of these short-chain compounds were on the rise since the adoption of the Montreal Protocol; Young’s team is the first to verify this by analyzing yearly levels of these compounds in ice cores, going back to 1990.
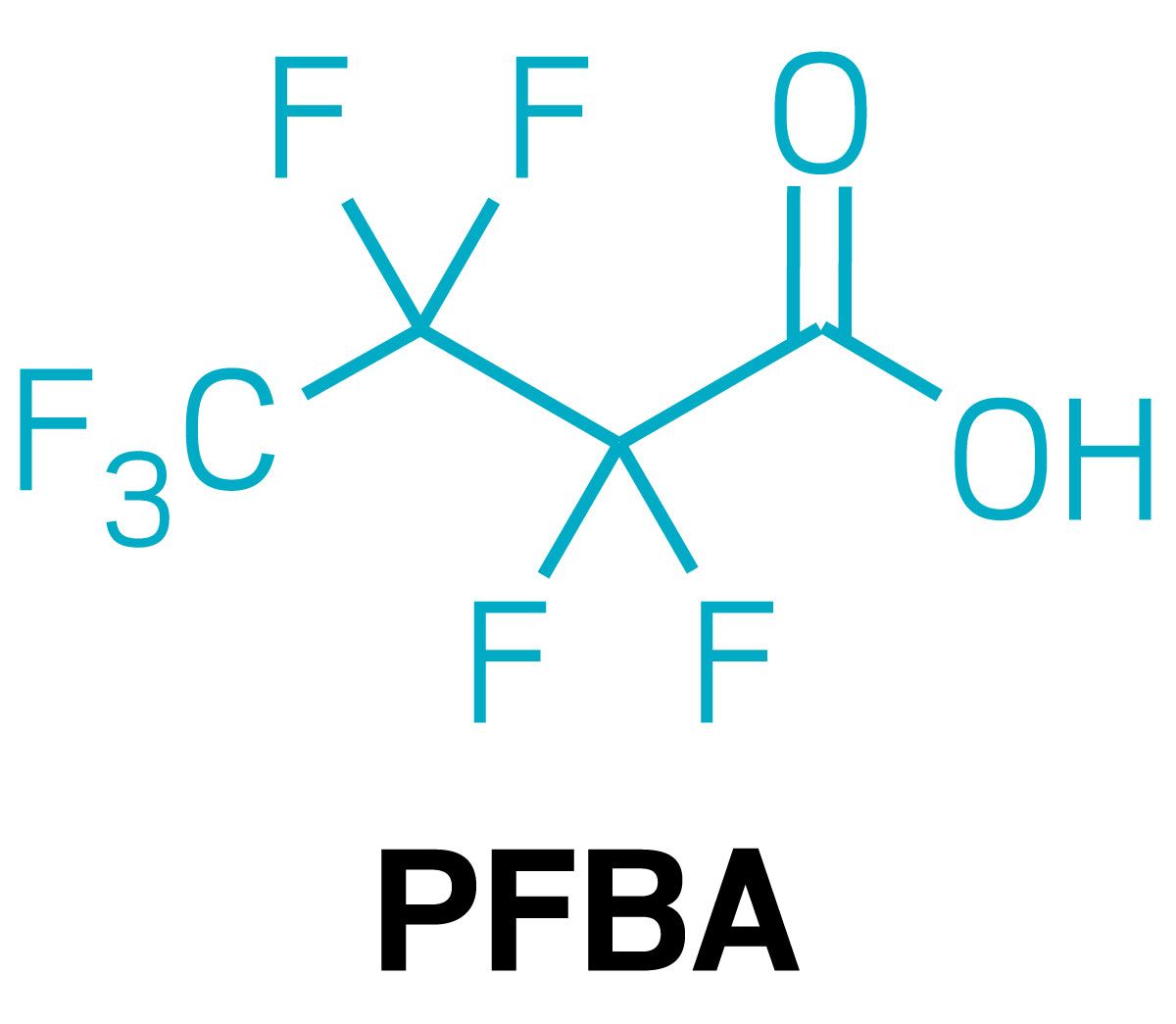
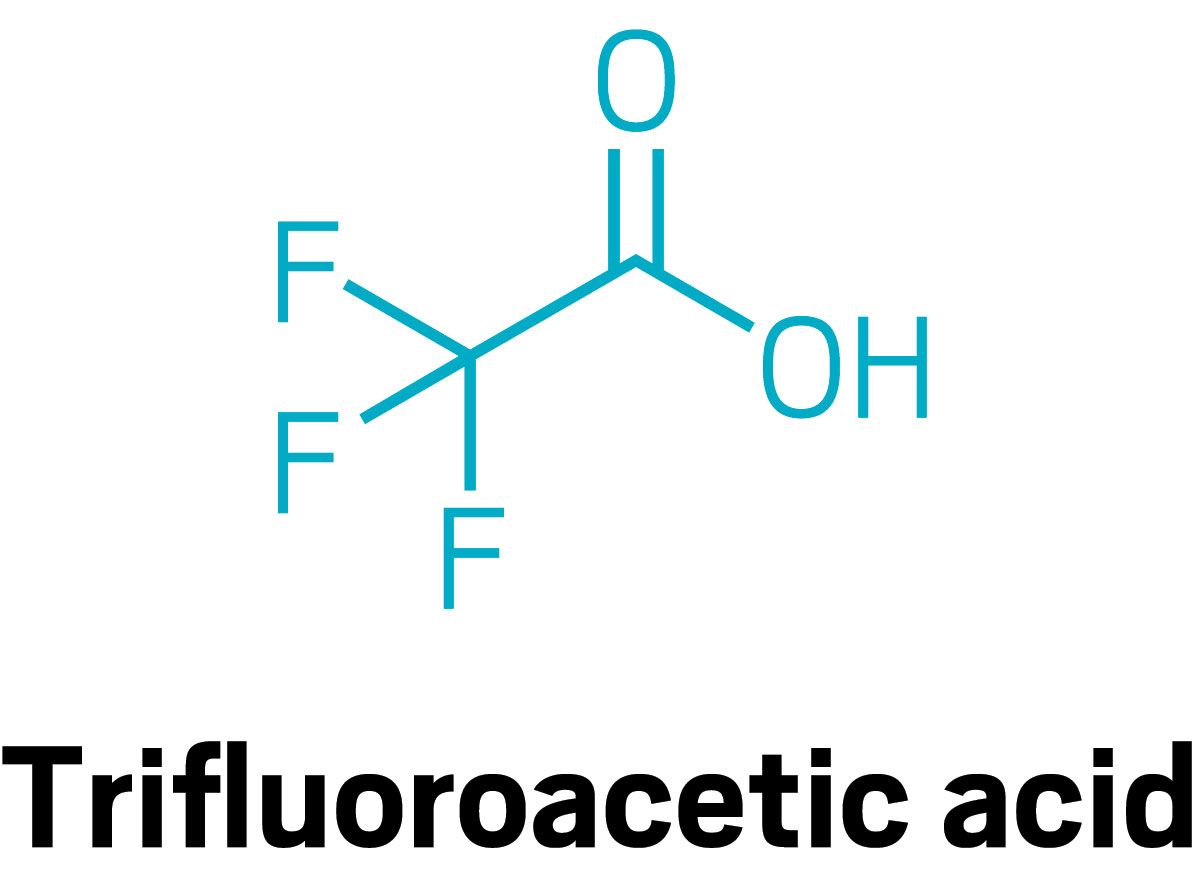
Young and Amila De Silva, a chemist at Environment and Climate Change Canada, focused on three of the smallest compounds in this class using ice cores drilled by University of Alberta glaciologist Alison Criscitiello. Each layer in an ice core represents a year of snowfall, and preserves a representative sample of what was in the atmosphere that year. Young and De Silva measured yearly levels of three PFAS in ice cores from the Devon Ice Cap in Nunavut, Canada: trifluoroacetic acid (TFA), perfluorobutanoic acid (PFBA), and perfluoropropionic acid (PFPrA). Then they used atmospheric modeling to try to deduce the sources of the compounds.
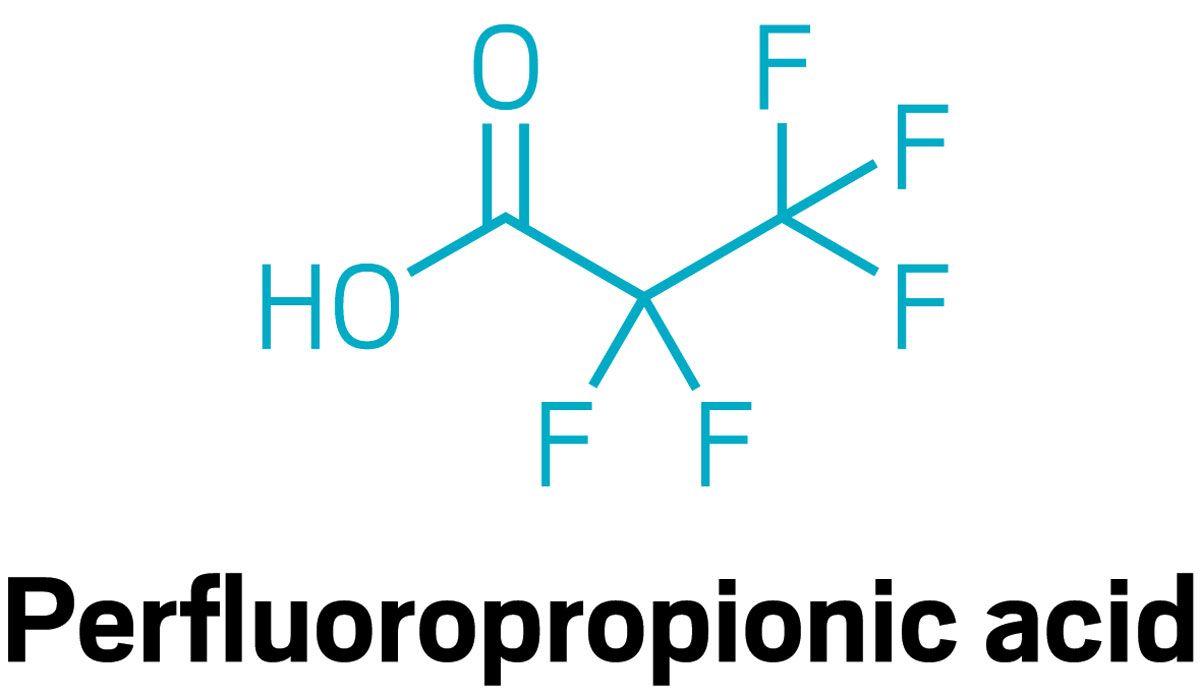
Levels of all three compounds have been increasing since 1990. Their modeling work—which uses information about chemical production, meteorology, and the atmospheric chemistry of fluoropolymers—ties two of the compounds to CFC replacements. TFA is a byproduct of hydrofluorocarbons used in vehicle air conditioners. One, the CFC replacement HFC-134a, is a strong greenhouse gas, and is currently being phased out by another, called HFO-1234yf, which has no climate impact. However, HFO-1234yf breaks down into far more TFA than the compound it’s replacing, Young says. As a result, Young expects TFA levels in the Arctic will continue to increase. Young’s analysis suggests PFBA is also a byproduct of CFC replacements. But her team was unable to pinpoint the source of PFPrA.
“Very little is known about the environmental toxicity of these short-chain acids,” says P. Lee Ferguson, an environmental chemist at Duke University who is not involved with the project. Most research on PFAS in the environment has focused on long-chain compounds because they are easier to measure and they are well known for accumulating in the environment. The shorter compounds are highly soluble, so they are difficult to separate using the usual PFAS analysis techniques, such as liquid chromotography/mass spectrometry. In the new study, Young used ion-exchange chromatography to separate out the elusive compounds.
Though not as well known, these smaller compounds still pose an environmental concern. “There’s been a resurgence of interest” in them, Young says. Earlier this year, researchers found high levels of TFA in human blood (Environ. Int. 2020, DOI: 10.1016/j.envint.2019.105295). And multiple studies have shown that short-chain PFAS can accumulate in wetlands and in plants, including food crops, particularly those grown in hydroponic systems, Young says.
These particular compounds mix rapidly in the atmosphere, so it is hard to pinpoint the countries that are emitting them, Young says. But, she says, they are likely circulating throughout the Northern Hemisphere. The fact that these highly mobile compounds have made their way to the Devon Ice Cap means they’re probably also being deposited in more populous regions to the south, where people may be exposed to them in the air or in their drinking water.
If these compounds turn out to be toxic to people and other living things, that could be a big problem, Young says, as they’re already in the environment at high levels. Kimberley Rain Miner, who studies glacial pollution at the University of Maine Climate Change Institute, thinks more work needs to be done to study the potential toxicity and sources of the short-chain compounds found in this new study.
Young says her research points to a problem chemists have been grappling with for decades. “Reliance on performance chemicals is not without cost,” she says. “When you replace CFCs with something else, you’re kicking the can down the road, and changing the problem,” not eliminating it.
Chemical & Engineering News
ISSN 0009-2347
Copyright © 2020 American Chemical Society